Sn Element
Periodic Table
0
Symbol
Sn 0
Group Number
14 4
Period Number
5 3
Block
p block 0
Element Family
Post-Transition 0
CAS Number
7440315 50
Space Group Name
I41/amd 0
Space Group Number
141.00 7
Facts
0
Interesting Facts
- In the list of most abundant element Tin is ranked 49th.
- Tin metal does not react with water as well as does not corrode in it.
Sources
Found in Minerals, Mining 0
History
0
Who Discovered
Unknown 0
Discovery
Before 3500 BC 0
Abundance
0
Abundance In Universe
4 * 10-7 % 20
Abundance In Sun
~0.0000009 % 19
Abundance In Meteorites
0.00 % 24
Abundance In Earth's Crust
0.00 % 34
Abundance In Oceans
0.00 % 26
Abundance In Humans
0.00 % 13
Uses
0
Uses & Benefits
- Tin-niobium alloy is used for producing superconducting magnets.
- Tin salt known as a tin II chloride, it is used as a mordant and as a reducing agent for dyeing calico and silk.
Industrial Uses
Automobile Industry, Chemical Industry, Food Industry 0
Medical Uses
Dentistry 0
Other Uses
NA 0
Biological Properties
0
Toxicity
Non Toxic 0
Present in Human Body
Yes 0
In Blood
0.38 Blood/mg dm-3 10
In Bone
1.40 p.p.m. 16
Physical
0
Melting Point
231.90 °C 72
Boiling Point
2,270.00 °C 48
Appearance
0
Physical State
Solid 0
Color
Silvery White 0
Luster
NA 0
Hardness
0
Mohs Hardness
1.50 18
Brinell Hardness
50.00 MPa 48
Vickers Hardness
Not Available 0
Speed of Sound
2,730.00 m/s 32
Optical Properties
0
Refractive Index
Not Available 0
Reflectivity
Not Available 0
Allotropes
Yes 0
α Allotropes
Grey Tin (alpha Tin, Tin Pest) 0
β Allotropes
White Tin (Beta Tin) 0
γ Allotropes
Rhombic Tin (gamma Tin) 0
Chemical
0
Chemical Formula
Sn 0
Isotopes
0
Known Isotopes
35 4
Electronegativity
0
Pauling Electronegativity
1.96 8
Sanderson Electronegativity
1.49 15
Allred Rochow Electronegativity
1.72 4
Mulliken-Jaffe Electronegativity
2.21 3
Allen Electronegativity
1.82 11
Electropositivity
0
Pauling Electropositivity
2.04 45
Ionization Energies
0
1st Energy Level
708.60 kJ/mol 33
2nd Energy Level
1,411.80 kJ/mol 48
3rd Energy Level
2,943.00 kJ/mol 30
4th Energy Level
3,930.30 kJ/mol 41
5th Energy Level
7,456.00 kJ/mol 13
6th Energy Level
Not Available 0
7th Energy level
Not Available 0
8th Energy Level
Not Available 0
9th Energy Level
Not Available 0
10th Energy Level
Not Available 0
11th Energy Level
Not Available 0
12th Energy Level
Not Available 0
13th Energy Level
Not Available 0
14th Energy Level
Not Available 0
15th Energy Level
Not Available 0
16th Energy Level
Not Available 0
17th Energy Level
Not Available 0
18th Energy Level
Not Available 0
19th Energy Level
Not Available 0
20th Energy Level
Not Available 0
21st Energy Level
Not Available 0
22nd Energy Level
Not Available 0
23rd Energy Level
Not Available 0
24th Energy Level
Not Available 0
25th Energy Level
Not Available 0
26th Energy Level
Not Available 0
27th Energy Level
Not Available 0
28th Energy Level
Not Available 0
29th Energy Level
Not Available 0
30th Energy Level
Not Available 0
Electrochemical Equivalent
1.11 g/amp-hr 55
Electron Work Function
4.42 eV 16
Other Chemical Properties
Ionization, Solubility 0
Atomic
0
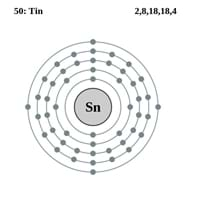
Atomic Number
50 62
Electron Configuration
[Kr] 4d10 5s2 5p2 0
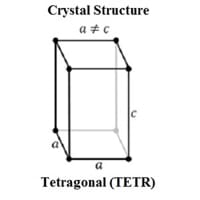
Crystal Structure
Tetragonal (TETR) 0
Crystal Lattice
TETR-Crystal-Structure-of-Tin.jpg#100 0
Atom
0
Number of Protons
50 61
Number of Neutrons
69 44
Number of Electrons
50 61
Radius of an Atom
0
Atomic Radius
140.00 pm 38
Covalent Radius
139.00 pm 45
Van der Waals Radius
217.00 pm 23
Atomic Weight
118.71 amu 56
Atomic Volume
16.30 cm3/mol 32
Adjacent Atomic Numbers
0
Previous Element
43 0
Next Element
40 0
Valence Electron Potential
83.50 (-eV) 14
Lattice Constant
583.18 pm 7
Lattice Angles
π/2, π/2, π/2 0
Lattice C/A Ratio
Not Available 0
Mechanical
0
Density
0
Density At Room Temperature
7.37 g/cm3 54
Density When Liquid (at m.p.)
6.99 g/cm3 34
Tensile Strength
Not Available 0
Viscosity
Not Available 0
Vapor Pressure
0
Vapor Pressure at 1000 K
0.00 (Pa) 17
Vapor Pressure at 2000 K
Not Available 0
Elasticity properties
0
Shear Modulus
18.00 GPa 36
Bulk Modulus
58.00 GPa 20
Young's Modulus
50.00 GPa 36
Poisson Ratio
0.36 8
Other Mechanical Properties
Ductile, Malleable 0
Magnetic
0
Magnetic Characteristics
0
Specific Gravity
7.31 42
Magnetic Ordering
Diamagnetic 0
Permeability
Not Available 0
Susceptibility
Not Available 0
Electrical Properties
0
Electrical Property
Superconductor 0
Resistivity
115.00 nΩ·m 28
Electrical Conductivity
0.09 106/cm Ω 23
Electron Affinity
107.30 kJ/mol 8
Thermal
0
Specific Heat
0.23 J/(kg K) 28
Molar Heat Capacity
27.11 J/mol·K 20
Thermal Conductivity
66.80 W/m·K 25
Critical Temperature
Not Available 0
Thermal Expansion
22.00 µm/(m·K) 20
Enthalpy
0
Enthalpy of Vaporization
290.40 kJ/mol 32
Enthalpy of Fusion
7.03 kJ/mol 47
Enthalpy of Atomization
301.30 kJ/mol 35
Standard Molar Entropy
51.20 J/mol.K 29
|
||
|
||
|