Cu Element
Periodic Table
0
Symbol
Cu 0
Group Number
11 7
Period Number
4 4
Block
d block 0
Element Family
Transition Metal 0
CAS Number
7440508 37
Space Group Name
Fm_ 3m 0
Space Group Number
225.00 2
Facts
0
Interesting Facts
- Copper rarely found in its pure form in nature.
- Copper Sulfate is mostly used in agricultural poison and as an algicide in water purification system.
Sources
Found in Minerals 0
History
0
Who Discovered
Unknown 0
Discovery
In Middle East (9000 BCE) 0
Abundance
0
Abundance In Universe
6 * 10-6 % 12
Abundance In Sun
~0.00007 % 12
Abundance In Meteorites
0.01 % 13
Abundance In Earth's Crust
0.01 % 17
Abundance In Oceans
0.00 % 8
Abundance In Humans
0.00 % 9
Uses
0
Uses & Benefits
- It is use for coinage and bullion.
- Most of copper element is used in manufacturing electrical and electronic equipments such as wirings and components. It is also used in construction and industrial machine.
Industrial Uses
Chemical Industry, Electronic Industry 0
Medical Uses
NA 0
Other Uses
Alloys, Coinage, Jewellery 0
Biological Properties
0
Toxicity
Non Toxic 0
Present in Human Body
Yes 0
In Blood
1.01 Blood/mg dm-3 8
In Bone
26.00 p.p.m. 12
Physical
0
Melting Point
1,084.62 °C 40
Boiling Point
2,562.00 °C 43
Appearance
0
Physical State
Solid 0
Color
Copper 0
Luster
NA 0
Hardness
0
Mohs Hardness
3.00 12
Brinell Hardness
235.00 MPa 38
Vickers Hardness
343.00 MPa 28
Speed of Sound
3,810.00 m/s 19
Optical Properties
0
Refractive Index
Not Available 0
Reflectivity
90.00 % 3
Allotropes
No 0
α Allotropes
Not Available 0
β Allotropes
Not Available 0
γ Allotropes
Not Available 0
Chemical
0
Chemical Formula
Cu 0
Isotopes
0
Known Isotopes
29 10
Electronegativity
0
Pauling Electronegativity
1.90 11
Sanderson Electronegativity
1.98 9
Allred Rochow Electronegativity
1.75 3
Mulliken-Jaffe Electronegativity
1.49 14
Allen Electronegativity
1.85 9
Electropositivity
0
Pauling Electropositivity
2.10 42
Ionization Energies
0
1st Energy Level
745.50 kJ/mol 22
2nd Energy Level
1,957.90 kJ/mol 15
3rd Energy Level
3,555.00 kJ/mol 15
4th Energy Level
5,536.00 kJ/mol 12
5th Energy Level
7,700.00 kJ/mol 11
6th Energy Level
9,900.00 kJ/mol 10
7th Energy level
13,400.00 kJ/mol 7
8th Energy Level
16,000.00 kJ/mol 9
9th Energy Level
19,200.00 kJ/mol 9
10th Energy Level
22,400.00 kJ/mol 11
11th Energy Level
25,600.00 kJ/mol 13
12th Energy Level
35,600.00 kJ/mol 6
13th Energy Level
38,700.00 kJ/mol 6
14th Energy Level
42,000.00 kJ/mol 7
15th Energy Level
46,700.00 kJ/mol 9
16th Energy Level
50,200.00 kJ/mol 10
17th Energy Level
53,700.00 kJ/mol 12
18th Energy Level
61,100.00 kJ/mol 10
19th Energy Level
64,702.00 kJ/mol 11
20th Energy Level
163,700.00 kJ/mol 2
21st Energy Level
174,100.00 kJ/mol 2
22nd Energy Level
184,900.00 kJ/mol 1
23rd Energy Level
198,800.00 kJ/mol 1
24th Energy Level
Not Available 100
25th Energy Level
Not Available 100
26th Energy Level
Not Available 100
27th Energy Level
Not Available 100
28th Energy Level
Not Available 100
29th Energy Level
Not Available 100
30th Energy Level
Not Available 0
Electrochemical Equivalent
1.19 g/amp-hr 53
Electron Work Function
4.65 eV 11
Other Chemical Properties
Chemical Stability, Corrosion, Ionization, Solubility 0
Atomic
0
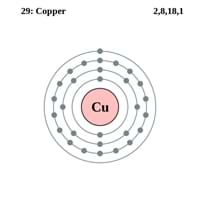
Atomic Number
29 78
Electron Configuration
[Ar] 3d10 4s1 0
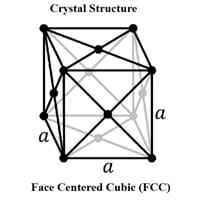
Crystal Structure
Face Centered Cubic (FCC) 0
Crystal Lattice
FCC-Crystal-Structure-of-Copper.jpg#100 0
Atom
0
Number of Protons
29 77
Number of Neutrons
35 58
Number of Electrons
29 77
Radius of an Atom
0
Atomic Radius
128.00 pm 46
Covalent Radius
132.00 pm 48
Van der Waals Radius
140.00 pm 44
Atomic Weight
63.55 amu 72
Atomic Volume
7.10 cm3/mol 61
Adjacent Atomic Numbers
0
Previous Element
22 0
Next Element
21 0
Valence Electron Potential
34.00 (-eV) 50
Lattice Constant
361.49 pm 35
Lattice Angles
π/2, π/2, π/2 0
Lattice C/A Ratio
Not Available 0
Mechanical
0
Density
0
Density At Room Temperature
8.96 g/cm3 43
Density When Liquid (at m.p.)
8.02 g/cm3 27
Tensile Strength
Not Available 0
Viscosity
Not Available 0
Vapor Pressure
0
Vapor Pressure at 1000 K
1.53 (Pa) 8
Vapor Pressure at 2000 K
Not Available 0
Elasticity properties
0
Shear Modulus
48.00 GPa 15
Bulk Modulus
140.00 GPa 12
Young's Modulus
120.00 GPa 17
Poisson Ratio
0.34 10
Other Mechanical Properties
Ductile, Malleable 0
Magnetic
0
Magnetic Characteristics
0
Specific Gravity
8.89 34
Magnetic Ordering
Diamagnetic 0
Permeability
1.256629 * 10-6 H/m 5
Susceptibility
-9.63 * 10-6 4
Electrical Properties
0
Electrical Property
Conductor 0
Resistivity
16.78 nΩ·m 50
Electrical Conductivity
0.60 106/cm Ω 2
Electron Affinity
222.80 kJ/mol 1
Thermal
0
Specific Heat
0.38 J/(kg K) 15
Molar Heat Capacity
24.44 J/mol·K 51
Thermal Conductivity
401.00 W/m·K 2
Critical Temperature
Not Available 0
Thermal Expansion
16.50 µm/(m·K) 25
Enthalpy
0
Enthalpy of Vaporization
283.70 kJ/mol 33
Enthalpy of Fusion
7.11 kJ/mol 46
Enthalpy of Atomization
338.90 kJ/mol 29
Standard Molar Entropy
33.20 J/mol.K 45
|
||
|
||
|
|
||
|
||
|