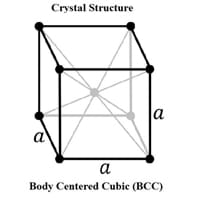
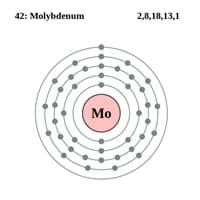
Chemical Properties of Molybdenum
Chemical
0
Chemical Formula
Mo 0
Isotopes
0
Known Isotopes
25 14
Electronegativity
0
Pauling Electronegativity
2.16 5
Sanderson Electronegativity
1.15 19
Allred Rochow Electronegativity
1.30 24
Mulliken-Jaffe Electronegativity
Not Available 0
Allen Electronegativity
2.16 3
Electropositivity
0
Pauling Electropositivity
1.84 48
Ionization Energies
0
1st Energy Level
684.30 kJ/mol 37
2nd Energy Level
1,560.00 kJ/mol 37
3rd Energy Level
2,618.00 kJ/mol 43
4th Energy Level
4,480.00 kJ/mol 26
5th Energy Level
5,257.00 kJ/mol 32
6th Energy Level
6,640.80 kJ/mol 22
7th Energy level
12,125.00 kJ/mol 13
8th Energy Level
13,860.00 kJ/mol 16
9th Energy Level
15,835.00 kJ/mol 16
10th Energy Level
17,980.00 kJ/mol 18
11th Energy Level
20,190.00 kJ/mol 17
12th Energy Level
22,219.00 kJ/mol 15
13th Energy Level
26,930.00 kJ/mol 13
14th Energy Level
29,196.00 kJ/mol 13
15th Energy Level
52,490.00 kJ/mol 7
16th Energy Level
55,000.00 kJ/mol 8
17th Energy Level
61,400.00 kJ/mol 9
18th Energy Level
67,700.00 kJ/mol 9
19th Energy Level
74,000.00 kJ/mol 9
20th Energy Level
80,400.00 kJ/mol 10
21st Energy Level
87,000.00 kJ/mol 9
22nd Energy Level
93,400.00 kJ/mol 7
23rd Energy Level
98,420.00 kJ/mol 6
24th Energy Level
104,400.00 kJ/mol 2
25th Energy Level
121,900.00 kJ/mol 1
26th Energy Level
127,700.00 kJ/mol 1
27th Energy Level
133,800.00 kJ/mol 1
28th Energy Level
139,800.00 kJ/mol 1
29th Energy Level
148,100.00 kJ/mol 1
30th Energy Level
154,500.00 kJ/mol 1
Electrochemical Equivalent
0.89 g/amp-hr 60
Electron Work Function
4.60 eV 12
Other Chemical Properties
Anti Corrosion, Ionization, Radioactive Isotopes, Solubility 0
|
||
|
||
|