V Element
Periodic Table
0
Symbol
V 0
Group Number
5 13
Period Number
4 4
Block
d block 0
Element Family
Transition Metal 0
CAS Number
7440622 27
Space Group Name
Im_ 3m 0
Space Group Number
229.00 1
Facts
0
Interesting Facts
- Vanadium is a highly reactive metal, hence it is not found free in nature.
- Vanadium can be found in almost 65 different types of minerals.
Sources
Found As a By-product, Found in Minerals, Mining, Ores of Minerals 0
History
0
Who Discovered
Andrés Manuel del Río 0
Discovery
In 1801 0
Abundance
0
Abundance In Universe
1 * 10-4 % 10
Abundance In Sun
~0.00004 % 13
Abundance In Meteorites
0.01 % 14
Abundance In Earth's Crust
0.02 % 12
Abundance In Oceans
0.00 % 14
Abundance In Humans
0.00 % 16
Uses
0
Uses & Benefits
- Its ally with steel is used very tough and hence it is used in armor plates, axles, piston rods, tools and crankshafts.
- Its oxide is used as pigments for glass and ceramics.
Industrial Uses
Aerospace Industry, Automobile Industry, Chemical Industry 0
Medical Uses
NA 0
Other Uses
Alloys 0
Biological Properties
0
Toxicity
Toxic 0
Present in Human Body
Yes 0
In Blood
0.00 Blood/mg dm-3 36
In Bone
0.00 p.p.m. 31
Physical
0
Melting Point
1,890.00 °C 13
Boiling Point
3,380.00 °C 20
Appearance
0
Physical State
Solid 0
Color
Blue-Silver Grey 0
Luster
Metallic 0
Hardness
0
Mohs Hardness
6.70 4
Brinell Hardness
600.00 MPa 20
Vickers Hardness
628.00 MPa 17
Speed of Sound
4,560.00 m/s 17
Optical Properties
0
Refractive Index
Not Available 0
Reflectivity
61.00 % 14
Allotropes
No 0
α Allotropes
Not Available 0
β Allotropes
Not Available 0
γ Allotropes
Not Available 0
Chemical
0
Chemical Formula
V 0
Isotopes
0
Known Isotopes
22 17
Electronegativity
0
Pauling Electronegativity
1.63 20
Sanderson Electronegativity
1.39 17
Allred Rochow Electronegativity
1.45 16
Mulliken-Jaffe Electronegativity
Not Available 0
Allen Electronegativity
1.53 26
Electropositivity
0
Pauling Electropositivity
2.37 34
Ionization Energies
0
1st Energy Level
650.90 kJ/mol 42
2nd Energy Level
1,414.00 kJ/mol 47
3rd Energy Level
2,830.00 kJ/mol 35
4th Energy Level
4,507.00 kJ/mol 25
5th Energy Level
6,298.70 kJ/mol 23
6th Energy Level
12,363.00 kJ/mol 5
7th Energy level
14,530.00 kJ/mol 5
8th Energy Level
16,730.00 kJ/mol 7
9th Energy Level
19,860.00 kJ/mol 7
10th Energy Level
22,240.00 kJ/mol 12
11th Energy Level
24,670.00 kJ/mol 15
12th Energy Level
29,730.00 kJ/mol 12
13th Energy Level
32,446.00 kJ/mol 12
14th Energy Level
86,450.00 kJ/mol 1
15th Energy Level
94,170.00 kJ/mol 2
16th Energy Level
102,300.00 kJ/mol 3
17th Energy Level
112,700.00 kJ/mol 4
18th Energy Level
121,600.00 kJ/mol 5
19th Energy Level
130,700.00 kJ/mol 6
20th Energy Level
143,400.00 kJ/mol 8
21st Energy Level
151,440.00 kJ/mol 8
22nd Energy Level
Not Available 100
23rd Energy Level
Not Available 100
24th Energy Level
Not Available 0
25th Energy Level
Not Available 0
26th Energy Level
Not Available 0
27th Energy Level
Not Available 0
28th Energy Level
Not Available 0
29th Energy Level
Not Available 0
30th Energy Level
Not Available 0
Electrochemical Equivalent
0.38 g/amp-hr 71
Electron Work Function
4.30 eV 18
Other Chemical Properties
Anti Corrosion, Ionization, Radioactive Isotopes 0
Atomic
0
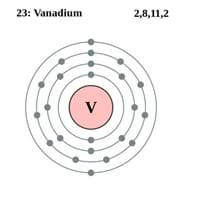
Atomic Number
23 83
Electron Configuration
[Ar] 3d3 4s2 0
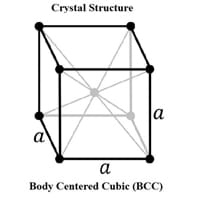
Crystal Structure
Body Centered Cubic (BCC) 0
Crystal Lattice
BCC-Crystal-Structure-.jpg#100 0
Atom
0
Number of Protons
23 82
Number of Neutrons
28 61
Number of Electrons
23 82
Radius of an Atom
0
Atomic Radius
134.00 pm 43
Covalent Radius
153.00 pm 33
Van der Waals Radius
200.00 pm 28
Atomic Weight
50.94 amu 77
Atomic Volume
8.78 cm3/mol 56
Adjacent Atomic Numbers
0
Previous Element
11 0
Next Element
29 0
Valence Electron Potential
120.00 (-eV) 6
Lattice Constant
303.00 pm 58
Lattice Angles
π/2, π/2, π/2 0
Lattice C/A Ratio
Not Available 0
Mechanical
0
Density
0
Density At Room Temperature
6.00 g/cm3 66
Density When Liquid (at m.p.)
5.50 g/cm3 46
Tensile Strength
800.00 MPa 6
Viscosity
Not Available 0
Vapor Pressure
0
Vapor Pressure at 1000 K
Not Available 0
Vapor Pressure at 2000 K
0.23 (Pa) 16
Elasticity properties
0
Shear Modulus
47.00 GPa 16
Bulk Modulus
160.00 GPa 11
Young's Modulus
128.00 GPa 15
Poisson Ratio
0.37 7
Other Mechanical Properties
Ductile, Malleable 0
Magnetic
0
Magnetic Characteristics
0
Specific Gravity
5.96 53
Magnetic Ordering
Paramagnetic 0
Permeability
Not Available 0
Susceptibility
Not Available 0
Electrical Properties
0
Electrical Property
Superconductor 0
Resistivity
197.00 nΩ·m 19
Electrical Conductivity
0.05 106/cm Ω 35
Electron Affinity
50.60 kJ/mol 20
Thermal
0
Specific Heat
0.49 J/(kg K) 10
Molar Heat Capacity
24.89 J/mol·K 46
Thermal Conductivity
30.70 W/m·K 39
Critical Temperature
Not Available 0
Thermal Expansion
8.40 µm/(m·K) 46
Enthalpy
0
Enthalpy of Vaporization
458.60 kJ/mol 14
Enthalpy of Fusion
17.57 kJ/mol 14
Enthalpy of Atomization
514.60 kJ/mol 13
Standard Molar Entropy
28.90 J/mol.K 52
|
||
|
||
|